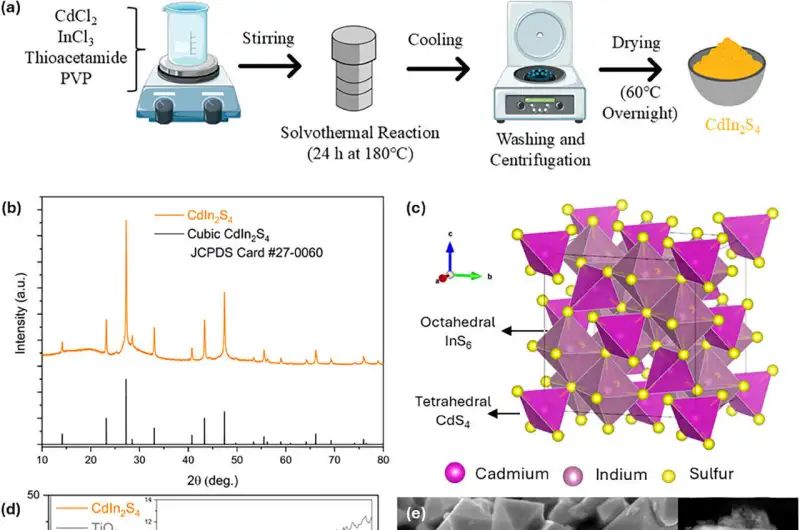
 Image Source: Phys.org
Image Source: Phys.org
In a significant advance for environmental remediation, scientists at the University of Adelaide have developed a novel sunlight-activated material capable of degrading per- and polyfluoroalkyl substances (PFAS) — notoriously persistent "forever chemicals" — into harmless components including fluoride. This innovative technology represents a promising, energy-efficient solution to PFAS contamination in water and offers hope for large-scale environmental cleanup and safer communities.
Key Highlights: Degrading the Indestructible PFAS
The research team engineered a catalyst that works under sunlight to break down PFAS chemicals by targeting their strong carbon-fluorine bonds, which until now have made PFAS extremely resistant to degradation.
Unlike traditional methods that struggle due to PFAS molecular protection, this sunlight-driven process focuses on disrupting the fluorine atoms protecting the carbon chains, resulting in the complete breakdown of these compounds.
The degradation process produces fluoride ions as a byproduct, which can be safely isolated and repurposed for uses such as healthcare products like toothpaste or as fertilizer additives.
The breakthrough offers a low-energy, scalable approach that could be integrated into water treatment systems to efficiently remove PFAS from contaminated water sources.
Understanding PFAS and Their Challenges
PFAS are a group of synthetic chemicals widely used in products ranging from non-stick cookware and water-repellent fabrics to firefighting foams.
Their unique chemical structure, especially the strong carbon-fluorine bonds, leads to remarkable stability, causing PFAS to accumulate persistently in the environment and living organisms.
Exposure to PFAS has been linked to serious health concerns including developmental issues, infertility, and certain cancers.
More than 85% of people in countries like Australia have measurable PFAS levels in their blood, leading regulatory bodies to dramatically lower safe drinking water limits for these chemicals to nanogram per liter levels.
Technology and Mechanism of Action
The novel catalyst activates under solar light to produce reactive species that break the carbon-fluorine bonds via a process optimized to degrade the fluorine protective shield.
This approach bypasses the challenges faced by conventional treatments that usually focus on carbon atoms and rarely succeed in full PFAS destruction.
The technology leverages sunlight, making it both environmentally sustainable and cost-effective compared to energy-intensive current methods.
Ongoing research aims to improve catalyst stability and efficiency for application in large water treatment plants.
Potential Applications and Environmental Impact
This material could be deployed in PFAS-treatment chains where PFAS-contaminated water is first captured and concentrated, then exposed to the sunlight-activated catalyst for degradation.
It promises to revolutionize management of PFAS pollution from diverse sources such as industrial wastewater, firefighting sites, and contaminated drinking water supplies.
By breaking down PFAS to safe, reusable fluoride, the technology prevents recontamination risks associated with mere filtration or storage methods.
The solution offers the prospect of restoring ecosystems affected by PFAS and protecting public health globally.
Expert Insights and Future Directions
Dr. Cameron Shearer, lead researcher at the University of Adelaide, emphasizes this work as a critical step toward safer communities and cleaner ecosystems through effective PFAS remediation.
Further projects are focused on scaling up the process, enhancing long-term catalyst durability, and integrating sensing technologies for real-time monitoring of PFAS degradation.
The scientific community views this innovation as part of a broader push for green, efficient remediation technologies addressing persistent organic pollutants.
Conclusion
The sunlight-activated catalyst developed by the University of Adelaide researchers offers a groundbreaking, energy-efficient method to conquer the longstanding environmental and health challenges posed by PFAS "forever chemicals." This approach not only achieves complete degradation but also recovers valuable fluoride, signaling a sustainable future for water safety and environmental restoration. As PFAS contamination continues to threaten ecosystems and human health worldwide, this technology paves the way for practical, scalable clean-up strategies driven by renewable solar energy.
Sources: University of Adelaide Newsroom, Phys.org
Advertisement
Advertisement






